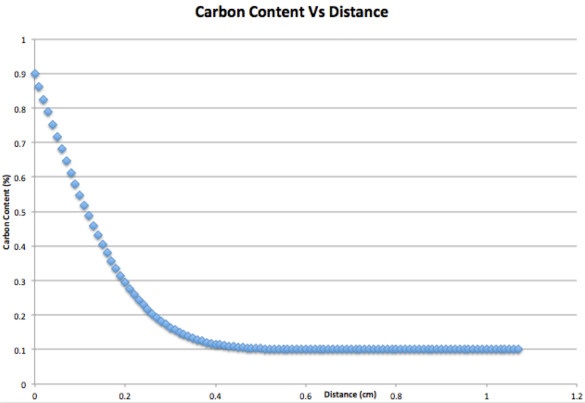
For a 0.1% carbon steel, being carburised at 920 degrees celsius, plot the carbon content as a function of distance below the surface, after 15 hours. The diffusion coefficient is 2.72×10^-7cm^2s^-1 and carbon potential of the gas is 0.9%.
What is the maximum distance below the surface that the carbon has diffused to?
Co = 0.1%
Cs = 0.9%
Dc = 2.72×10^-7cms^-1
t = 54000s
| X values (cm) | erf (Y) | Cx (%) |
| 0 | 0 | 0.9 |
| 0.01 | 0.046526179 | 0.862779057 |
| 0.02 | 0.092894291 | 0.825684568 |
| 0.03 | 0.138947876 | 0.788841699 |
| 0.04 | 0.184533669 | 0.752373065 |
| 0.05 | 0.22950312 | 0.716397504 |
| 0.06 | 0.273713851 | 0.681028919 |
| 0.07 | 0.317030995 | 0.646375204 |
| 0.08 | 0.359328423 | 0.612537262 |
| 0.09 | 0.400489821 | 0.579608143 |
| 0.1 | 0.440409617 | 0.547672307 |
| 0.11 | 0.478993728 | 0.516805017 |
| 0.12 | 0.516160149 | 0.487071881 |
| 0.13 | 0.551839338 | 0.45852853 |
| 0.14 | 0.585974443 | 0.431220446 |
| 0.15 | 0.618521335 | 0.405182932 |
| 0.16 | 0.64944848 | 0.380441216 |
| 0.17 | 0.678736644 | 0.357010684 |
| 0.18 | 0.706378456 | 0.334897235 |
| 0.19 | 0.732377829 | 0.314097737 |
| 0.2 | 0.756749272 | 0.294600582 |
| 0.21 | 0.779517104 | 0.276386317 |
| 0.22 | 0.800714576 | 0.259428339 |
| 0.23 | 0.820382954 | 0.243693637 |
| 0.24 | 0.838570538 | 0.22914357 |
| 0.25 | 0.855331675 | 0.21573466 |
| 0.26 | 0.870725764 | 0.203419388 |
| 0.27 | 0.884816268 | 0.192146985 |
| 0.28 | 0.897669759 | 0.181864193 |
| 0.29 | 0.909354999 | 0.172516001 |
| 0.3 | 0.91994208 | 0.164046336 |
| 0.31 | 0.929501617 | 0.156398707 |
| 0.32 | 0.938104013 | 0.149516789 |
| 0.33 | 0.945818804 | 0.143344957 |
| 0.34 | 0.952714066 | 0.137828747 |
| 0.35 | 0.95885592 | 0.132915264 |
| 0.36 | 0.9643081 | 0.12855352 |
| 0.37 | 0.969131607 | 0.124694714 |
| 0.38 | 0.973384433 | 0.121292453 |
| 0.39 | 0.977121359 | 0.118302913 |
| 0.4 | 0.980393811 | 0.115684952 |
| 0.41 | 0.983249783 | 0.113400173 |
| 0.42 | 0.985733813 | 0.111412949 |
| 0.43 | 0.987887 | 0.1096904 |
| 0.44 | 0.989747067 | 0.108202346 |
| 0.45 | 0.991348458 | 0.106921233 |
| 0.46 | 0.992722464 | 0.105822029 |
| 0.47 | 0.993897365 | 0.104882108 |
| 0.48 | 0.9948986 | 0.10408112 |
| 0.49 | 0.995748942 | 0.103400847 |
| 0.5 | 0.996468676 | 0.102825059 |
| 0.51 | 0.997075794 | 0.102339365 |
| 0.52 | 0.997586177 | 0.101931058 |
| 0.53 | 0.998013781 | 0.101588975 |
| 0.54 | 0.998370814 | 0.101303349 |
| 0.55 | 0.99866791 | 0.101065672 |
| 0.56 | 0.998914292 | 0.100868566 |
| 0.57 | 0.999117922 | 0.100705662 |
| 0.58 | 0.999285647 | 0.100571483 |
| 0.59 | 0.999423328 | 0.100461338 |
| 0.6 | 0.999535962 | 0.10037123 |
| 0.61 | 0.999627795 | 0.100297764 |
| 0.62 | 0.999702412 | 0.100238071 |
| 0.63 | 0.999762835 | 0.100189732 |
| 0.64 | 0.999811598 | 0.100150722 |
| 0.65 | 0.999850817 | 0.100119347 |
| 0.66 | 0.999882253 | 0.100094198 |
| 0.67 | 0.999907365 | 0.100074108 |
| 0.68 | 0.999927357 | 0.100058114 |
| 0.69 | 0.999943219 | 0.100045425 |
| 0.7 | 0.999955762 | 0.100035391 |
| 0.71 | 0.999965645 | 0.100027484 |
| 0.72 | 0.999973407 | 0.100021274 |
| 0.73 | 0.999979483 | 0.100016414 |
| 0.74 | 0.999984222 | 0.100012623 |
| 0.75 | 0.999987905 | 0.100009676 |
| 0.76 | 0.999990759 | 0.100007392 |
| 0.77 | 0.999992963 | 0.10000563 |
| 0.78 | 0.999994659 | 0.100004273 |
| 0.79 | 0.999995959 | 0.100003233 |
| 0.8 | 0.999996953 | 0.100002438 |
| 0.81 | 0.99999771 | 0.100001832 |
| 0.82 | 0.999998284 | 0.100001373 |
| 0.83 | 0.999998719 | 0.100001025 |
| 0.84 | 0.999999046 | 0.100000763 |
| 0.85 | 0.999999293 | 0.100000566 |
| 0.86 | 0.999999477 | 0.100000418 |
| 0.87 | 0.999999615 | 0.100000308 |
| 0.88 | 0.999999717 | 0.100000226 |
| 0.89 | 0.999999793 | 0.100000166 |
| 0.9 | 0.999999849 | 0.100000121 |
| 0.91 | 0.99999989 | 0.100000088 |
| 0.92 | 0.99999992 | 0.100000064 |
| 0.93 | 0.999999942 | 0.100000046 |
| 0.94 | 0.999999959 | 0.100000033 |
| 0.95 | 0.99999997 | 0.100000024 |
| 0.96 | 0.999999979 | 0.100000017 |
| 0.97 | 0.999999985 | 0.100000012 |
| 0.98 | 0.999999989 | 0.100000009 |
| 0.99 | 0.999999992 | 0.100000006 |
| 1 | 0.999999995 | 0.100000004 |
| 1.01 | 0.999999996 | 0.100000003 |
| 1.02 | 0.999999997 | 0.100000002 |
| 1.03 | 0.999999998 | 0.100000001 |
| 1.04 | 0.999999999 | 0.100000001 |
| 1.05 | 0.999999999 | 0.100000001 |
| 1.06 | 0.999999999 | 0.1 |
| 1.07 | 1 | 0.1 |